
Nuclear fusion only takes at high temperature in the order of 10 7 to 10 9 K and high pressure to push hydrogen nuclei closer to fuse. Where M–Mass, E–Energy, C–Velocity of light (3 x 10 8 ms -1 ). The relation between energy and mass proposed by Einstein is E = MC 2. This can be converted into energy and vice versa. This mass is converted to energy, as per the mass-energy equivalence. This difference is mass is called Mass Defect. The mass of the daughter nuclei formed during fission and fusion reaction is less than the sum of the masses of the two-parent nuclei. The average energy released in each fusion reaction is 3.84 x 10 -12 J.Īlpha rays, positrons, and Neutrino are emitted. 1 H 2 represents an isotope of hydrogen called ‘Deuterium’. Example: 1 H 2 + 1 H 2 à 2 He 4 + Q (Energy). This phenomenon is called Nuclear Fusion. If two lighter nuclei joined to create a heavier nucleus. The Nuclear bomb dropped in Nagasaki was called Fat Man, it was an explosion-type bomb that used Plutonium core. The Nuclear bomb dropped in Hiroshima is called the Little Boy of Gun Type bomb that used Uranium Core. Atom Bomb or Nuclear bombĪtom Bomb is the classical example of an uncontrolled chain reaction that happens in a small interval of time leading to a huge explosion. If the fissile material is more than the critical mass, we call it Supercritical. If the fissile material is less than the Critical Mass, we call it Subcritical. This value is called Critical Mass (Mc).Īs per the above statement, Critical Mass is the minimum mass of the fissile material to maintain the chain reaction. This can be done only when the size of the fissionable material is at a certain optimum range. To maintain the chain reaction the number of neutrons produced must be greater than the number of neutrons lost. If it loses these neutrons, the chain reaction cannot continue further. Some neutrons are lost called leakage of neutrons, and some are absorbed by the non-fissionable materials. Not all the neutrons are released to go for a chain reaction. Critical Massĭuring the fission process, it releases about 2 to 3 neutrons. As a result, enormous Neutrons were produced and continued to multiply at a very rapid rate resulting in the release of an enormous amount of energy. There the Neutron release allowed to multiply rapidly with no control. In the Uncontrolled Chain Reaction, it does not control the neutrons released. This controlled chain reaction is used for a constructive purpose such as Nuclear Power Generation. The excess neutron produced in the nuclear reaction is absorbed by the Neutron absorber. Only one neutron is allowed to do the chain reaction. In the controlled chain reaction, Only one neutron is allowed to release. One is a Controlled Chain Reaction, and Another one is an Uncontrolled Chain reaction. A Chain reaction is a process in which the number of neutrons multiplies rapidly. In process continuous and rapidly multiplying Neutrons in a geometrical progression.

Uranium nucleus similar producing 27 Neutrons. These three neutrons again react with the three other nuclei of Uranium producing nine neutrons. Chain ReactionĪ Uranium nucleus U-235, when bombarded with a neutron, goes through fission by producing three neutrons. Example of Fertile material is Uranium-238, Thorium-232, Plutonium-240. We can convert some radioactive material into fissionable material. All isotopes of Uranium do not go through Nuclear fission when hit by a neutron.Įxample U 235 is fissionable, U 238 is not fissionable.

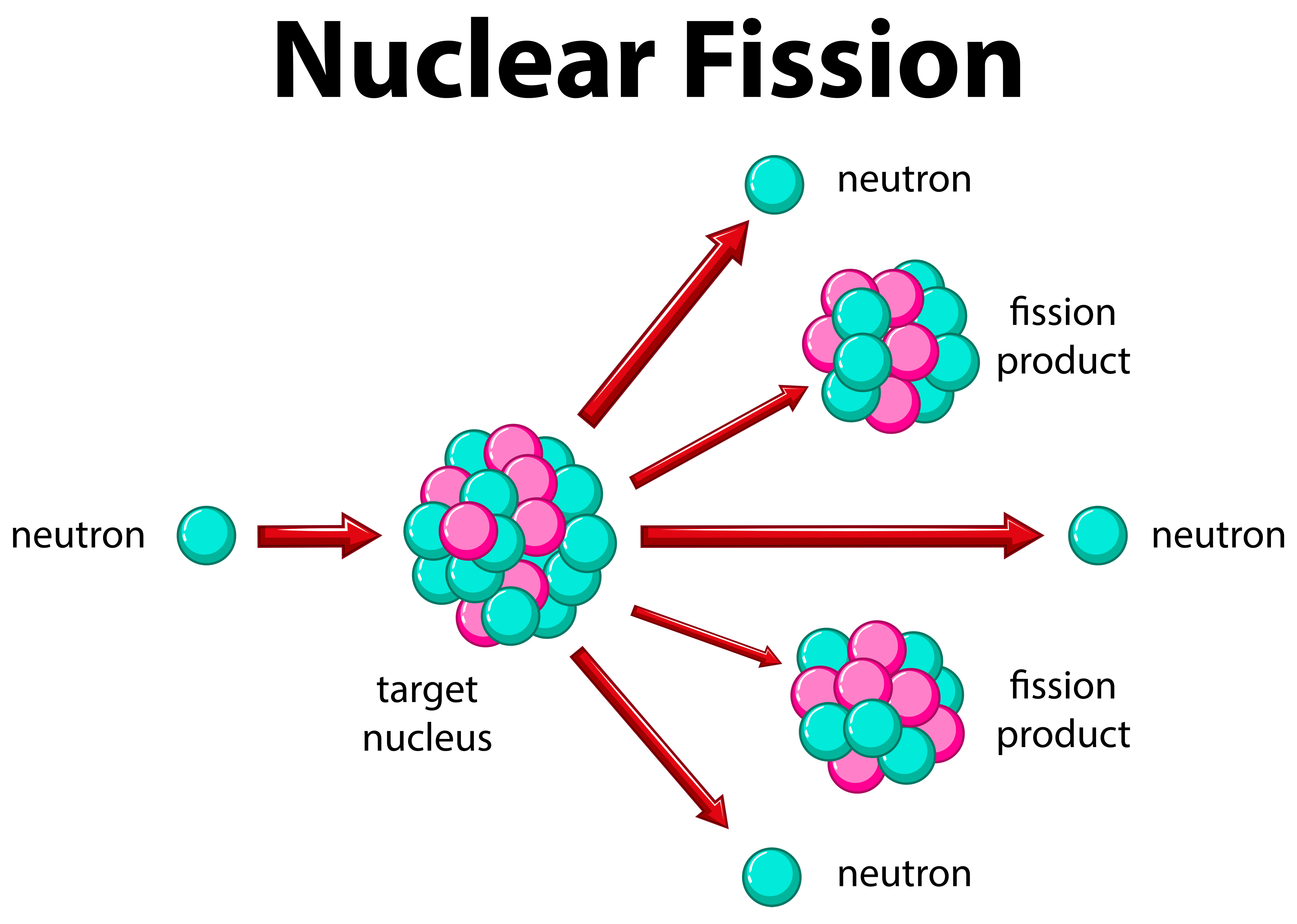
Fissionable MaterialsĪ Fissionable material also called Fissile Material, is a radioactive element, that goes through fission in a constant process when it is hit by a neutron. Fission reaction that emits Gamma radiation, creates mutation in the human gene and causes genetic diseases. Alpha, Beta, and Gamma radiations are emitted. We can perform nuclear Fission at room temperature. The average energy released in fission is 3.2 x 10 -11 J. Nuclear fission is the phenomenon of splitting the heavier nucleus into two smaller nuclei with the release of an enormous amount of energy and a few neutrons are called Nuclear Fission.Įxample: Nuclear fission of a Uranium Nucleus (U235).ĩ2 U 235 + 0 n 1 à56 Ba 141 + 36 Kr 92 + 3 0 n 1 + Q (Energy) German scientists, Otto Hahn, and F.Strassman in 1939 discovered that when Uranium Nucleus is bombarded with a neutron, it splits into two smaller Nuclei among with emission of neutrons and energy.


 0 kommentar(er)
0 kommentar(er)
